Contents:
- Explain how different wavelengths of light interact with matter, including the concept of resonance, different types of transitions, chromophores/auxochromes, and selection rules.
- Draw and label a Jablonski energy level diagram and relate its features to absorption spectra.
- Describe the processes that occur when UV-visible light is absorbed by a molecule, including excited states and relaxation processes.
- Explain the differences between atomic and molecular absorption spectra.
- Explain the significance of the terms in the Beer-Lambert Law, and how they relate to molecular features and experimental design.
- Explain the limitations of Beer’s Law.
- Utilize Beer’s Law for determining the concentration of one or more analyte species from one or more absorbance measurements.
- Relate the choice and arrangement of components in a spectrophotomer to its capabilities, performance, advantages and disadvantages. Draw and label a detailed block diagram of a spectrophotometer, including the light path and components, and describe the function of each of those components.
- Describe the operation of key components for measuring absorbance:
- misc notes
Explain how different wavelengths of light interact with matter, including the concept of resonance, different types of transitions, chromophores/auxochromes, and selection rules.
- resonance: energy matching
- quantum yield: fraction of excited states that do emit photon
- chromophore: light absorbing groups (part of molecule)
- 200 - 800 nm: pi-electron and hetero atoms with non-bonding valence shell electron pairs
- non bonding electrons in water, alcohols, either don’t absorb above 160nm, they are suitable solvents for spectroscopy
- increasing/extending conjugation of unsaturated bonds decreases transition energies -> energy gap between HOMO and LUMO decreases
- auxochrome: chemical group attached to chromophore that modifies its light absorption (alter energy of MOs)
- acid-base indicators: isobestic point -> total absorbance stays the same (?)
- non-boding electrons in O of water, alcohol, ether are good solvents for UV/Vis (don’t absorb above 160)
| Nature of Shift | Term | How |
|---|---|---|
| longer wavelength | red shift | adding double bonds |
| shorter wavelength | blue shift | |
| greater absorption | hyperchromic | doubles with each new conjugated double bond |
| lower absorption | hypochromic |
- HOMO LUMO transitions:
- in the UV/Vis range
- singlet state:
- ground: two electrons opposing spin, same orbital
- excited: two electrons opposing spin, different orbitals
Draw and label a Jablonski energy level diagram and relate its features to absorption spectra.
- Bandwidths also depend on local environment (including effect of vib. & rotational levels)
- we get absorption bands from electronic transitions, vibrational levels, rotational levels, other collections/interaction
Describe the processes that occur when UV-visible light is absorbed by a molecule, including excited states and relaxation processes.
- Relaxation pathways: fluorescence, internal conversion, intersystem crossing, phosphorescence
- vibrational relaxation: energy lost to heat, electron stays in same electronic state
- internal conversion: energy lost to heat, electron moves to lower electronic state, requires overlap between vibration levels and lower electronic state (horizontal energy transfer)
- fluorescence: only occurs in singlet to singlet state
- intersystem crossing: excited singlet move to excited triplet (or other way)
- phosphorescence: only occurs in triplet to singlet state
- light emitted from fluorescence or phosphorescence is always same or less than excitation wavelength
- relative time frame:
- absorption < vibration relaxation and internal conversion < fluorescence < phosphorescence (slow)
Explain the differences between atomic and molecular absorption spectra.
UV/Vis spectra for molecules/ions
- difference in energy between HOMO and LUMO -> UV/Vis, absorption of photon is possible
- types of transitions, n is non bonding:
- σ → σ*, 200 nm
- n → σ*, 160-260 nm
- π → π*, 200-500 nm
- n → π*, 250-600 nm
- charge transfer: inorganic metal-ligand complexes, electron from metal transferred to ligand -> produce very large absorbance
- UV/Vis more broad than IR
- UV/Vis absorption results in change to electronic energy levels and maybe vibrational -> number of closely spaced absorption bands that merge together to form single broad absorption band
- IR absorption only results in change to vibrational energy levels
UV/Vis spectra for atoms
- enough energy to cause change in atom’s valence electrons
- only allowed between $l +-1$$
- excited state lifetime is short
- narrow width in absorption lines, due to fixed difference in energy and lack of rotational/vibrational energy levels (width is 10−5 − 10−3 nm)
Explain the significance of the terms in the Beer-Lambert Law, and how they relate to molecular features and experimental design.
- light absorption: by chromophores, reflection, scattering loss
- molar absorption coefficient + path length => slope => sensitivity
Explain the limitations of Beer’s Law.
- real deviations:
- high concentration: chromophores affect charge distribution of other chromophores
- epsilon dependson refractive index
- apparent deviations:
- light is not perfectly monochromatic
Utilize Beer’s Law for determining the concentration of one or more analyte species from one or more absorbance measurements.
- mixtures: Atotal = A1 + A2 + ... = b(ϵ1c1 + ϵ2c2) + ...
- Aλ1 = b(ϵ1λ1c1 + ϵ2λ1c2) + ..., Aλ2 = b(ϵ1λ2c1 + ϵ2λ2c2) + ...
- etc for the other wavelengths
Relate the choice and arrangement of components in a spectrophotomer to its capabilities, performance, advantages and disadvantages. Draw and label a detailed block diagram of a spectrophotometer, including the light path and components, and describe the function of each of those components.
single beam instrument
- light source
- D (UV)
- T (Vis)
- glass absorbs wavelength less than 350 nm, quartz absorbs below 190 nm
- wavelength selector
- diffraction grating, prism, filter
- diffraction grating equation: a − b = mλ = d(sin(i) + sin(r))
- where:
- d: groove spacing (related to lines/mm)
- i: incident angle
- r: reflection angle
- m: order (use filters to block unwanted)
- where:
- diffraction grating equation: a − b = mλ = d(sin(i) + sin(r))
- monochromator: slit, diffraction grating, slit
- narrow slit => S/N decreases, but bandwidth more narrow
- double monochomator: reduce stray light + improve linearity => throughput decreased
- does not remove unwanted orders (need filter)
- slit => grating => slit => grating => slit
- groove density increases (lines/mm) => dispersion increases => resolution increases => working range decreases (PDA detector)
- diffraction grating, prism, filter
- Sample cell
- Photodetector
- PMT
- Photodiode array
- Computer
—
- UV/Vis diode array design
- dual lamp
- lens
- shutter
- sample
- lens
- slit
- grating
- PDA
- double beam: improve precision + S/N
- sample and reference cell
- compensate for flicker in lamp intensity
Describe the operation of key components for measuring absorbance:
Light sources (tungsten, deuterium)
- D (UV)
- T (Vis)
Monochromators (gratings and slits)
- filters have a fixed wavelength
- if we want to make measurements at different wavelengths -> need more than one filter
- monochromator: select narrow band of radiation, allow for continuous adjustment of band’s nominal wavelength
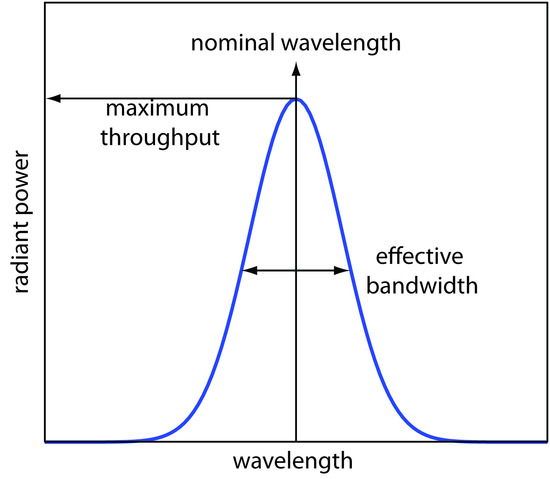
- nominal wavelength: the wavelength you want?
- want high throughput of radiation and narrow effective bandwidth
- nominal wavelength: the wavelength you want?
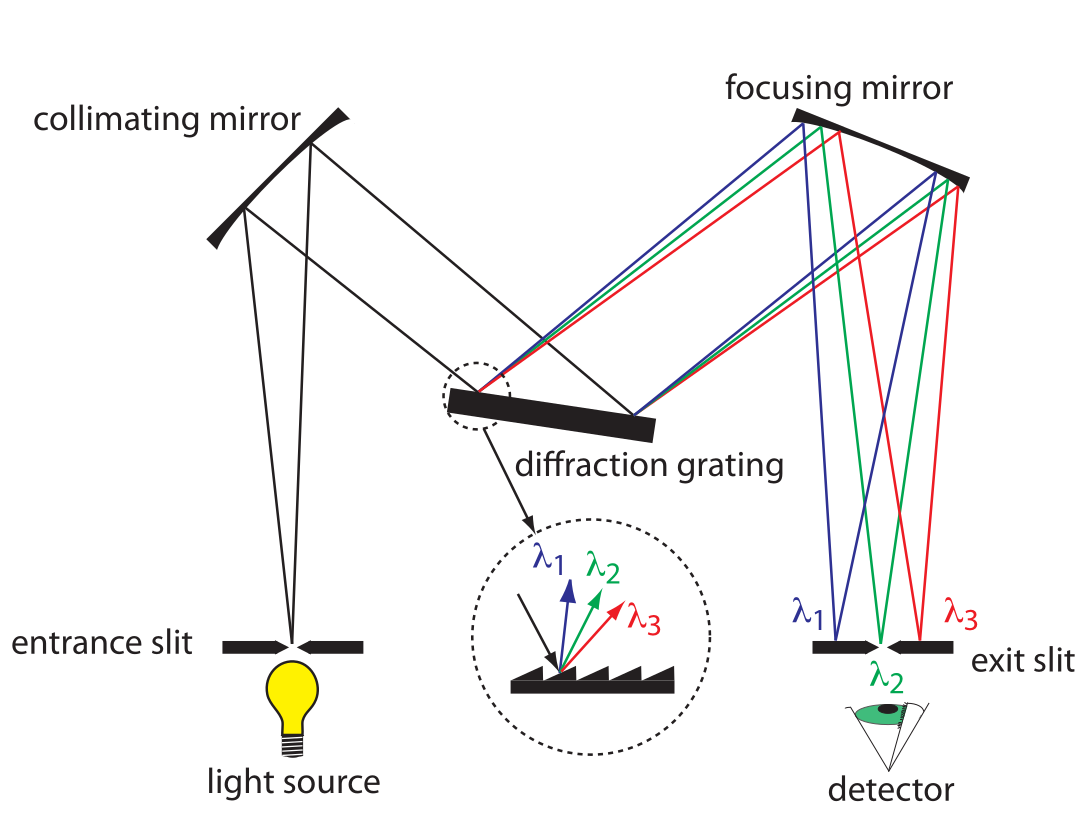
- collimating mirror: collects radiation
- reflects parallel beam of radiation to diffraction grating
- differaction grating: optically reflecting surface with large number of parallel grooves
- disperses radiation -> focused onto planar surface that contains exit slit
- or prism
- converts polychromatic source of radiation to monochromatic source of finite bandwidth
- exit slit:
- narrow: smaller effective bandwidth and better resolution, but smaller throughput of radiation
- can be fixed-wavelength or scanning
- fixed: manually select wavelength by rotating grating
Sample cells (design and materials)
- glass (silicate): 400 - 2000
- glass (optical): 350 - 2000
- quartz: UV 200 - 3000
- plastic: vis only, 380 - 780
Photodetectors (PMT, photodiode, photodiode arrays)
- not equally sensitive to all wavelengths
- measuring reference can help
- PMT
- photoelectric effect: signal amplification with electron cascade
- photocathode => focussing electrode => dynode(s) => anode
- more dynodes => more sensitive
- not equally sensitive to all wavelengths
- photoelectric effect: signal amplification with electron cascade
- silicon photodiode
- PN-junction in silicon
- voltage is created
- proportional to incident light intensity
- PDA (made of photodiode)
- entire spectrum measured
- PDA replaces exit slit => each PN element is a slit
- don’t have great S/N
misc notes
textbook notes:
- if energy (ℏv) of photon is more than excited state - ground state, excitation occurs
- atom/molecule in excited state can emit photon of energy ℏv
- you don’t see the colors a substance absorbs
spectroscopy based on absorption
- absorbed wavelength intensities are attenuated
- for an analyte to absorb EMR:
- there must be mechanism which EMR interacts with analyte -> UV/Vis changes energy of electrons, IR -> bond vibrational energy
- photon energy must equal different in energy between two allowed energy states
IR spectra for molecules for polyatomic molecules:
- energy for allowed vibration mode: $$E_v = v + \frac{1}{2} h v_o$$
- fundamental: +/- 1
- overtone: +/- 2,3
transmittance and absorbance
- transmittance: $$$T = \frac{P_T}{P_0}$
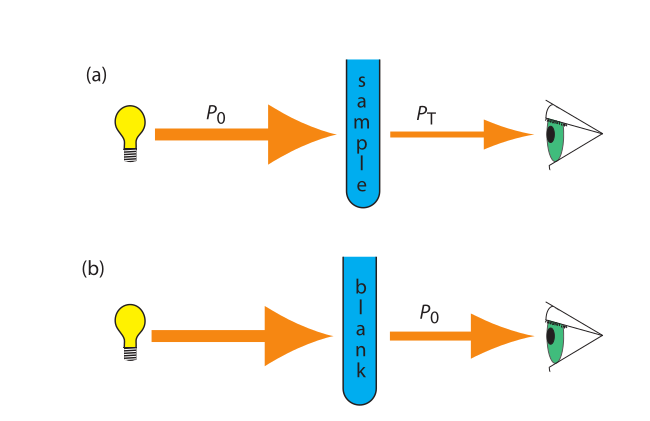
- redefine P0 from blank so we don’t need to care about loss of light from the source
- absorbance is linear function of analyte concentration: $$A = -log T = - log \frac{P_T}{P_0}$$
- require line source instead of continuum source because effective bandwidth is too large
- energy for allowed vibration mode: $$E_v = v + \frac{1}{2} h v_o$$
