Contents:
- Explain the role of the partition coefficient in separation and extraction techniques and describe the physical and chemical principles underlying separations.
- Describe and compare the different types and modes of chromatography with reference to the stationary and mobile phases and elution methods:
- Predict the order of elution of compounds with either normal or reversed phase chromatography.
- Interpret a simple chromatogram to identify the dead time, retention time, and calculate the partition coefficient, retention factor, and/or resolution.
- Describe the relationship between retention time, peak area, resolution and analytical figures of merit in quantitative and qualitative analysis using chromatography.
- Describe Van Deemter Equation and its applications in improving LC and GC separation performance
- Draw and label a block diagram of LC and GC systems and explain the role of each component, the characteristics of columns, and the operation and capabilities of detectors.
Explain the role of the partition coefficient in separation and extraction techniques and describe the physical and chemical principles underlying separations.
- partition coefficient: $$K = \frac{[A]_1}{[A]_2}$$
- how the analyte distributes between two phases based on equilibrium constant
- chromatography: carry out many seperatation steps for mixtures
- a column filled with SP
- sample added in solvent (MP), poured into column
- analyte moves between the phases based on partition coefficient ($$K = \frac{[A]_{mob}}{[A]_{stat}}$$), eventually moving out of the column
- spending more time in SP means spending more time in the column
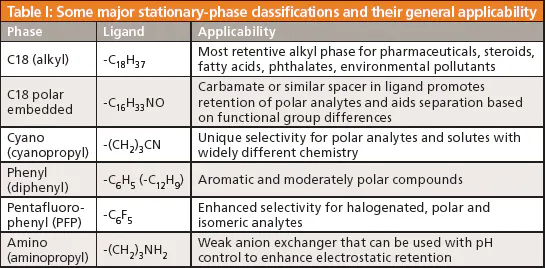
Describe and compare the different types and modes of chromatography with reference to the stationary and mobile phases and elution methods:
Adsorption vs. partition
- adsorption (on): only bind to surface of SP
- silica, alumina (SP)
- TLC: solvent (MP)
- column chromatography:
- column packed with silica/alumina
- elute with organic solvent as MP
- partition (in): solute dissolved in liquid phase
- HPLC
Reverse vs. normal phase
- reverse phase: MP is polar, SP is non polar (phenyl, C)
- analytes that are polar will elute faster (spend less time in SP)
- normal phase: MP is non polar (hexane, diethyl ether, ethyl acetate), SP is polar (silica, amino)
- analytes that are less polar will elute faster (spend less time in SP)
- analytes that are less polar will elute faster (spend less time in SP)
LC vs. GC
- LC:
- more applicable to any soluble compounds
- more versatile MP,SP
- compounds collected after
- less sample prep
- UV/Vis
- GC:
- require volatile/thermally stable compounds
- faster
- more sensitive, better resolution => more plates
- more universal detectors
- equipment less expensive
Isocratic/isothermal vs. gradient/temperature programming
LC
- general elution problem:
- more polar MP: poor late resolution
- more nonpolar MP: poor early resolution
- reverse phase: polar to nonpolar
- normal?
- gradient (programming) elution:
- isocratic: same elution throughout
- general elution problem:
GC
$$ u = \sqrt{\frac{B}{C}}$$
- temperature programming
Analytical vs. preparatory
- analytical: actual column that does separation
- preparatory: guard, shorter, same MP?
Predict the order of elution of compounds with either normal or reversed phase chromatography.
- reverse phase: MP is polar, SP is non polar (phenyl, C)
- analytes that are polar will elute faster (spend less time in SP)
- normal phase: MP is non polar (hexane, diethyl ether, ethyl acetate), SP is polar (silica, amino)
- analytes that are less polar will elute faster (spend less time in SP)
Interpret a simple chromatogram to identify the dead time, retention time, and calculate the partition coefficient, retention factor, and/or resolution.
- dead time: migration time, time for MP to travel the column
- retention time, adjusted retention time: time for analyte to elute from column
- retention factor: $$\frac{t_R - t_M}{t_M}$$
- resolution: occurs when more than 1.5
- $$\frac{2 (t_{RB} - t_{RA})}{(\Delta t)_B + (\Delta t)_A}$$, where (Δt)B is the width of the peak with tangents drawn (the bottom)
- $$\frac{1.178 (t_{RB} - t_{RA})}{(FWHM)_B + (FWHM)_A}$$, where (FWHM)B is the width at half height of the peak
Describe the relationship between retention time, peak area, resolution and analytical figures of merit in quantitative and qualitative analysis using chromatography.
- plate height: the more plates, the narrower the peak obtained
- $$N = \frac{16 t^2_R}{w^2}$$. where w is width, the longer time spent in column, the more plates, the wider the peak, the less plates
- resolution:
- $$\frac{\sqrt{N}}{4} \frac{\alpha - 1}{\alpha} \frac{k_B}{1 + k_B} = \frac{\sqrt{N}}{4} (1 - \frac{t_{RA}}{t_{RB}})$$, where $$\alpha = \frac{k_B}{k_A}$$ and B is retained longer
Describe Van Deemter Equation and its applications in improving LC and GC separation performance
- sources of band broadening:
- (A) eddy diffusion (multiple paths):
- solute take different paths through column packing
- smaller particle size => paths before more uniform, minimizing A
- diffusion length (distance needed to travel to SP) decreases, reducing C
- porous particles: better with larger overall particle sizes/lower pressures, lower C
- (B/v) longitudinal diffusion (based on concentration of analytes): diffusion from high concentrations to low concentrations
- (Cv) mass transfer effect: partitioning kinetics slow relative to MP flow rate (v), then peak is broadened
- if analyte moves in MP, the SP “catches up”, but the new eq has a broader width
- want: fast mass transfer relative to mobile phase velocity
- peak broadened: analyte diffusion is slow, or MP velocity is fast: can’t get close to eq
- if analyte moves in MP, the SP “catches up”, but the new eq has a broader width
- (A) eddy diffusion (multiple paths):
Draw and label a block diagram of LC and GC systems and explain the role of each component, the characteristics of columns, and the operation and capabilities of detectors.
LC
- components of HPLC:
- HPLC pump for liquid mobile phase
- (before) solvent reservoir
- injector for sample
- HPLC column
- (before) guard column
- (before) precolumn filter
- detector, where waste leaves after
- (after) back pressure regulator: prevents bubbles from forming in detector
- electronics, computer
- UV/Vis detector: Z-shape to offer longer path length that gives better sensitivity
- diode array: full spectra => speed
- UV/Vis detector: Z-shape to offer longer path length that gives better sensitivity
- HPLC pump for liquid mobile phase
- HPLC/UPLC
- HPLC* => HPLC => UPLC
- irregular => spherical =>
- decreasing size, increasing pressure
- HPLC column:
- bonded stationary phases: functional groups bonded to silica particles
GC
- temperature makes analytes go into gas phase
- analytes can be in vapour phase, condense in SP, dissolve in SP
- components of GC:
- mobile phase (gas cylinder): gas dry and free of impurities
- flow regulator
- injection system (temperature controlled)
- split: not all sample goes in
- splitless: all sample goes in
- column with oven (temperature controlled)
- detector
- flame ionization:
- signal proportionate to mass
- hydrocarbons burnt, no response to fully oxidized carbons
- universal, cheap, fast, dynamic range 107, detection low as: 10−12 g C/s
- flame ionization:
- capillary columns:
- little SP compared to LC
- need less analyte
- open tubular design: minimize multiple paths, A => 0, H minimized
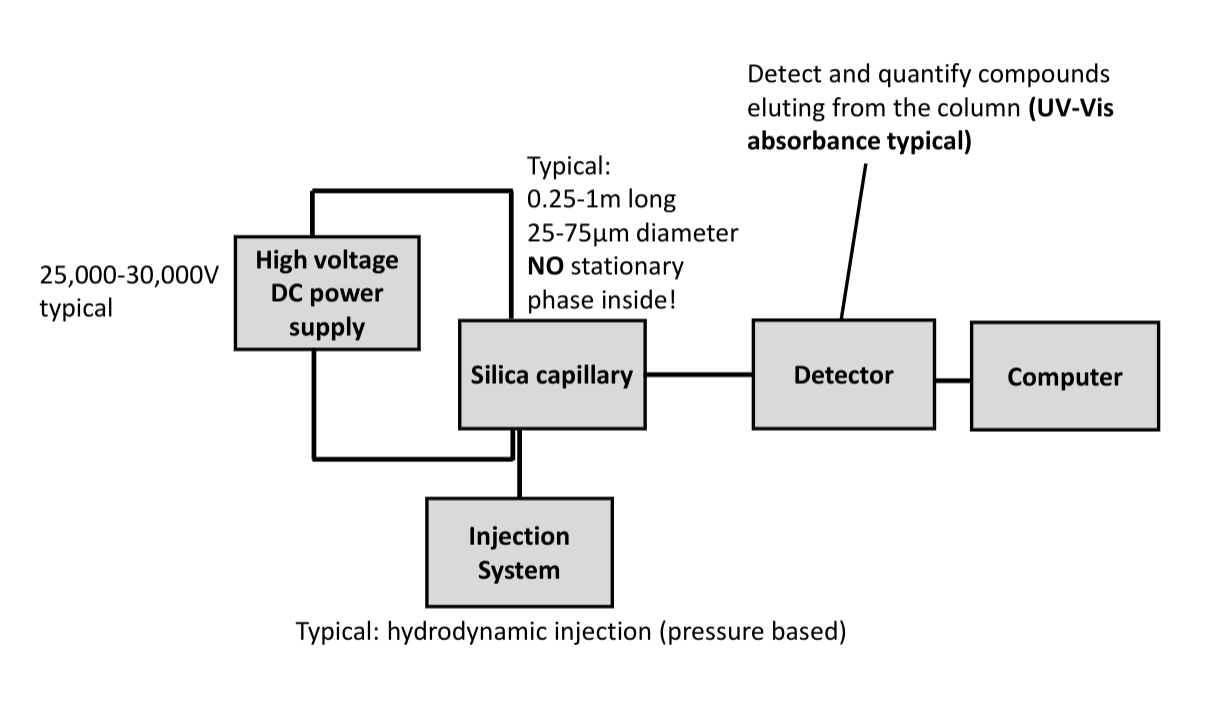
capillary electrophoresis
- separation of analytes based on making them move in electric field
- quantitative
- flow analytes through narrow capillary, past detector
- electrostatic driving force
- drag: friction
- as ion gets faster, drag force increases
- acceleration goes to zero as these forces balance
- eletrophoretic mobility: $$\mu_{ep} = \frac{q}{f}E = \mu_{ep}E$$
- bigger molecules, smaller mobility, because harder to push past solvent molecules
- higher charge: larger mobility, larger driving force
- solvent moves too
- μapp = μep + μeo, ion movement and solvent movement
- better resolution becase, A goes to zero (open tube), C goes to 0 since no SP, H is much smaller
textbook reading
12B
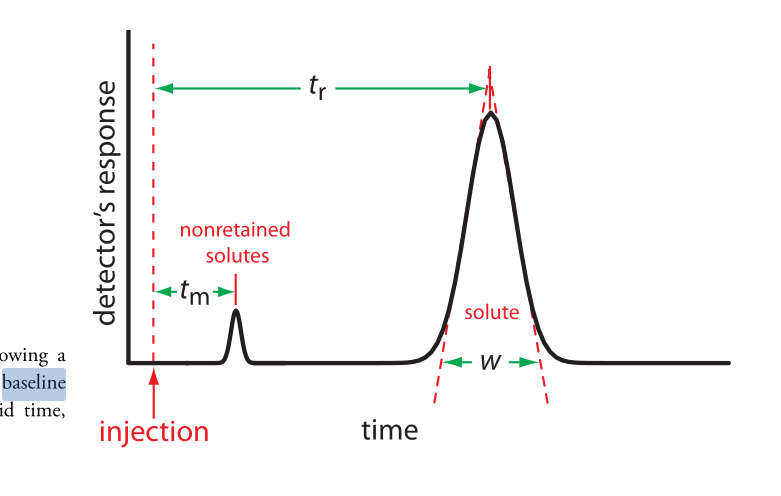
- characterize chromatographic peak: retention time, baseline width
- nonretained solutes: move through column at same rate as mobile phase => gives us void time (time to elute the nonretained solutes)
- resolution: measure of their separation: $$R_{AB} = \frac{t_{r, B} - t_{r, A}}{0.5 (w_B + w_A)} = 2 \frac{\Delta t_t}{w_B + w_A}$$, where B takes longer to elute
- improve by either increasing Δtt or decreasing wB + wA
- both solutes spend less time in mobile phase => retention factor
- increase selectivity => only one solute experience significant change in retention time
- baseline width: solutes movement within and between mobile phase/stationary phase => column efficiency
- improve by either increasing Δtt or decreasing wB + wA
solute retention factor
- the larger the retention factor, the more the distribution favors solute in stationary phase, and longer retention time
$$k = \frac{t_t - t_m}{t_m}$$
selectivity
- relative measure of retention of two solutes, $\alpha = \frac{k_B}{k_A}$, where A has smaller retention time
column efficiency
- quantitative measure of extent of band broadening: number of theoretical plates $N = \frac{L}{H}$, where L is column length and H is height of theoretical plate => more theoretical plates => chromatographic peaks become narrower
- theoretical model of chromatography => theoretical plates
- within each theoretical plate: eq between solute present in stationary phase and solute present in mobile phase
- given a Gaussian profile: $$H = \frac{\sigma^2}{L}$$
- width is 4 times the standard deviation
- gives us $$H = \frac{Lw^2}{16 t^2_r}$$
peak capacity
- estimate of number of solutes we can seperate
12C: optimizing chromatographic separations
- $$R_{AB} = \frac{t_{t, B} - t_{t, A}}{0.5 (w_B + w_A)}$$ or approximately equal $$\frac{t_{t, B} - t_{t, A}}{0.5 (2 w_B)} = \frac{t_{t, B} - t_{t, A}}{w_B}$$, where B is the later eluting of two solutes
- which we get $$R_{AB} = \frac{\sqrt{N_B}}{4} \times \frac{t_{t, B} - t_{t, A}}{t_{t, B}} = \frac{\sqrt{N_B}}{4} \times \frac{\alpha - 1}{\alpha} \times \frac{k_B - k_A}{1 + k_B}$$
using retention factor
- adjust B’s retention factor, kB, increasing will improve resolution, best if kB starts out being less than 10 => cost of longer analysis time
- to increase kB without changing α => nonselective increase to both retention factors
- GC: temperature (decrease)
- LC: weaker mobile phase solvent
- adjusting retention factors => too long of a retention time => general elution problem
- make adjustments to retention factor throughout separation
using selectivity
- if alpha is 1, not possible to improve resolution by adjusting solute retention factor or column efficiency
- to change, selectivity adjust solute retention factors
- if alpha is 1, not possible to improve resolution by adjusting solute retention factor or column efficiency
using column efficiency
- increase number of theoretical plates, N
- double N by doubling L, or cut the height
- contributions to band broadening:
- variations in path length (multiple paths)
- contribution to height of theoretical plate: Hp = 2λdp
- for column without packing material, Hp is zero and no contribution to band broadening from multiple paths
- more uniform packing material reduces this problem
- for column without packing material, Hp is zero and no contribution to band broadening from multiple paths
- contribution to height of theoretical plate: Hp = 2λdp
- longitudinal diffusion in mobile phase
- solute diffuses from high solute concentration to low solute concentration
- mass transfer in SP and MP
- movement between phases (mobile and stationary phase)
- band broadening occurs if solute’s movement within MP or SP is not fast enough to maintain an eq in its concentration between the two phases
- solute in MP moves down column before it passes into SP
- solute in SP moves takes longer than expected to move back into MP
- smaller velocity => more time for mass transfer
- variations in path length (multiple paths)
- increase number of theoretical plates, N
putting it all together
height of theoretical plate: H = Hp + Hd + Hs + Hm, where the contributions are path length, longitudinal diffusion, mass transfer in SP, mass transfer in MP
- another form is van deemter equation: H = A + B/u + Cu, where A is multiple paths, B/u accounts for longitudinal diffusion, Cu accounts for mass transfer
- to increase number of theoretical plates
- adjust velocity of mobile phase
- smaller mobile phase velocity: column efficiency limited by longitudinal diffusion
- higher mobile phase: two mass transfer terms
- column itself
- decrease particle size
- open-tubular or capillary columns
- small diameter
- no packing material
- interior is coated with SP
- Hp disappears, Hm decreases => H decreases
- takes less pressure to move down column => length increases
- difficult to inject reproducible
- adjust velocity of mobile phase
- use thin films of SP
- decrease Hs
- decreasing particle size (in SP):
- various number of paths become more uniform
- distance to get back into SP becomes smaller, so mass transfer decreases
- backpressure increases
- superficially porous particles => shorter diffusion length, minimize mass transfer, less pressure
- monolithic column: pack with one material, network of pores, less pressure
- lower pressure, higher flow rate, or make column longer
- $$R_{AB} = \frac{t_{t, B} - t_{t, A}}{0.5 (w_B + w_A)}$$ or approximately equal $$\frac{t_{t, B} - t_{t, A}}{0.5 (2 w_B)} = \frac{t_{t, B} - t_{t, A}}{w_B}$$, where B is the later eluting of two solutes
12D gas chromatography
gas MP
- carry solute through packed/capillary column, that separates sample components based on ability to partition between MP and SP
- components: compressed gas for MP, heated injector (volatilizes components in liquid sample), a column (in oven), detector
- packed column: larger sample
- filled with packing material
- glass rinsed to prevent adsorption of solute
- capillary column:
- better theoretical plate, longer
- smaller diameter, require smaller sample
gas SP
- elution order: boiling points of solutes/interaction between solutes and SP
- nonpolar solutes => more easy to seperate with nonpolar SP
- polar solutes => more easy to seperate with polar SP
- liquid SP: bleed, or tendency to elute when column is heated
sample introduction
- volatile
- appropriate concentration
- don’t degrade the separation (accidently injecting liquid sample or directly into moving stream of gas MP)
- packed: sample injected directly into column (mixes sample with least amount of carrier gas), heated above BP of least volatile solute
- capillary: need to use split/splitless
- split injection: injected into glass liner to mix with carrier gas
- only some gets injected, rest leaves
- splitless: allow all sample to enter column => significant precolumn band broadening is problem
- cool the column => solvent condences, traps solutes, then raise temperature back up
- split injection: injected into glass liner to mix with carrier gas
temperature control
- isothermal => set slightly below lowest boiling point solute
- causes high boiling point solute to have very long retention time => temperature programming
- detectors:
- thermal conductivity detector: measure thermal conductivity as MP exits column (universal detector)
- poor detection limit for most analytes
- flame ionization detector: combustion of organic compound in hydrogen gas flame => electrons and organic cations => suuply potential creates current => when amplified => signal
- many organic cations generate signal
- inorganic compounds not detected
- better detection limit
- electron capture detector
- highly selective towards solute electronegative functional groups
- insensitive to amines, alcohol, hydrocarbons
- excellent detection limit, but linear range only extend over two orders of magnitude
- mass spectrometer
- GCMS
- mass to charge ratio
- thermal conductivity detector: measure thermal conductivity as MP exits column (universal detector)
12E high performance liquid chromatography
- liquid MP
- components: reservoir for MP, pump for pushing MP through system, injector for introducing sample, column for separating the sample into component parts, detector for monitoring the eluent
HPLC columns
- analytical column: responsible for separation
- packed with silica
- capillary column use less solvent, can also be packed => back pressure that develops when pumping liquid
- monolithic column: solid support is single, porous rod
- guard column: placed beore sepration to protect from contamination
- solute that bind to SP (and wont come off)
- particulate material that clog
- same packing material and SP and shorter
- SP
- covalently bond to silica particles
- bonded stationary phases: reacting silica particles with organochlorosilane with R group (determines property of SP)
- normal phase: polar SP, nonpolar MP
- reverse phase: nonpolar SP, mobile MP
- MP
- elution order governed by polarity
- normal phase: less polar solutes elutes first
- reverse phase: more polar solute elutes first
- isocratic and gradient elutions
- move MP => dissolve gases, remove particulate matter => inject sample => loop injector
- detector: spectroscopic
- diode array
- UV/Vis detector
- detector: electrochemical
- refractive index, mass spec
- elution order governed by polarity
- comparison:
- loop injector: makes LC have better precision
- volume: LC has more
- LC not limited to volatile, can analyze broader range, applicable to any soluble compound, more versatile, can collect compounds after, less sample prep
- GC: more plates, can seperate more complex mixtures (higher resolution because more plates), faster, more universal detectors and less expensive
- no prep-scale GC because so narrow, but diameter so small so less mass transfer
12 F other forms
liquid-solid adsorption
- column packing is also SP
- SP is polar, MP is nonpolar
ion-exchange
- SP is cross-linked polymer resin with covalently attached ionic functional groups
size-exclusion
- ability of solute to enter pores of SP
key words
- adjusted retention time: t, = tt − tm, where tm is the time column’s void time, and tt is time between injection time and solute peak
- adsorption chromatography: flow solute over some surface, desired solute attaches onto the medium, not in the medium
- band broadening: when sample is injected => narrow band, and as sample passes through column, band broadens => band broadening
- column efficiency = extend of band broadening
- baseline width: extending tangent lines from inflection points on either side of peak through the baseline
- bleed: problem with liquid stationary phases, as temperature increases, is likely or possible to elute
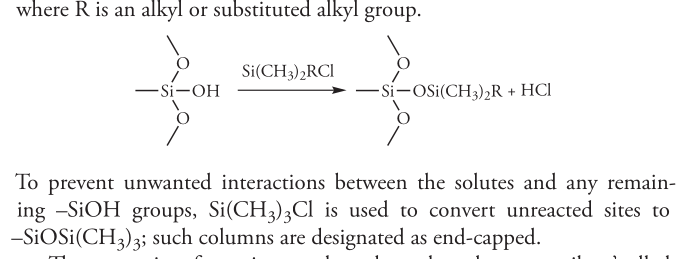
- bonded stationary phase: type of SP in capillary column, superior stability
- is attached chemically attached to the capillary’s silica surface
- reacting the silica particles with organochlorosilane, where the R group determines if polar or not polar
- capillary column: open tubular, very small diameter, contain no packing material
- interior coated with thin film of stationary phase
- plate height is reduced
- capillary electrochromatography
- capillary electrophoresis
- capillary gel electrophoresis
- capillary zone electrophoresis
- chromatogram chromatography
- column chromatography
- counter-current extraction
- cryogenic focusing
- electrokinetic injection
- electroosmotic flow
- electroosmotic flow velocity
- electron capture detector
- electropherogram
- electrophoresis
- electrophoretic mobility
- electrophoretic velocity
- exclusion limit
- flame ionization detector
- fronting
- gas chromatography
- gas–liquid chromatography
- gas–solid chromatography
- general elution problem
- guard column
- gradient elution: change mobile phase over time
- headspace sampling
- high-performance liquid chromatography
- hydrodynamic injection
- inclusion limition-exchange chromatography
- ion suppressor column
- ion exchange chromatography: separate analyte based on charge
- isocratic elution
- isothermal
- Joule heating
- Kovat’s retention index
- liquid–solid adsorption chromatography
- longitudinal diffusion
- loop injector
- mass spectrometer
- mass spectrum
- mass transfer
- micellemicellar electrokinetic capillary chromatography
- mobile phase
- monolithic column
- multiple paths
- nonretained solutes
- normal-phase chromatography
- on-column injection
- open tubular column
- packed columns
- partition chromatography
- peak capacity
- planar chromatography
- polarity index
- porous-layer open tubular column
- purge-and-trapresolution
- retention factor
- retention time
- reversed-phase chromatography
- selectivity factor
- single-column ion chromatography
- solid-phase microextraction
- split injection
- size exclusion chromatography: seperate analyte based on size
- splitless injection
- stacking
- stationary phase
- supercritical fluid chromatography
- support-coated open tubular column
- tailing
- temperature programming
- theoretical plate: column divided into sections, called plates, which with their own eq of solute in stationary phase and solute in mobile phase
- column efficiency = N = L/H
- H is height of a theoretical plate
- column efficiency improves, peaks become narrower, when there are more theoretical plates
- H = variance/length of column
- thermal conductivity detector
- van Deemter equation
- void time
- wall-coated open-tubular column
- zeta potential
